NVAF
Reducing stroke/SE risk*†

20 mg
Taken once daily with the evening meal in patients
with CrCl >50 mL/min
OR

15 mg
Taken once daily with the evening meal in patients with CrCl ≤50 mL/min
Tablets shown not actual size.
Renal dosing considerations
Periodically assess renal function as clinically indicated (ie, more frequently in situations in which renal function may decline) and adjust therapy accordingly. Consider dose adjustment or discontinuation of XARELTO® in patients who develop acute renal failure while on XARELTO®.
See section 8.6 of the Prescribing Information for additional information
Download PDF
*Calculate CrCl based on actual weight.
†Patients with CrCl <30 mL/min were not studied, but administration of XARELTO® is expected to result in serum concentrations of rivaroxaban similar to those in patients with moderate renal impairment (CrCl 30 mL/min to <50 mL/min).
§Eliquis® (apixaban) is a registered trademark of Bristol-Myers Squibb Company.
CrCl = creatinine clearance; NVAF = nonvalvular atrial fibrillation; SE = systemic embolism.
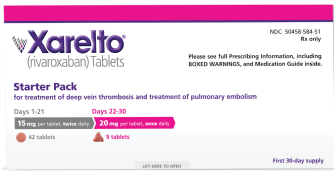
DVT/PE
For the treatment of
DVT and PE*†

15 mg
For the first 21 days
15 mg twice daily with food, at the same time each day, in patients with CrCl ≥15 mL/min
THEN

20 mg
Starting at day 22
Change to 20 mg once daily with food, at the same time each day, for remaining treatment in patients with CrCl ≥15 mL/min
Avoid use in patients with CrCl <15 mL/min
Tablets shown not actual size.


(after BID initiation period of 21 days)
carries a lower pill burden than Eliquis®1,2§
‡Taken with food.
Reduction in the risk
of DVT/PE recurrence*†

10 mg
10 mg once daily
Taken with or without food after ≥6 months of standard anticoagulant treatment in patients with CrCl ≥15 mL/min
Avoid use in patients with CrCl <15 mL/min
Tablet shown not actual size.
*Calculate CrCl based on actual weight.
†Patients with CrCl <30 mL/min were not studied, but administration of XARELTO® is expected to result in serum concentrations of rivaroxaban similar to those in patients with moderate renal impairment (CrCl 30 mL/min to <50 mL/min).
§Eliquis® (apixaban) is a registered trademark of Bristol-Myers Squibb Company.
§BID = twice daily; CrCl = creatinine clearance; DVT = deep vein thrombosis; PE = pulmonary embolism.
DVT prophylaxis
After hip replacement surgery*†

10 mg
10 mg once daily for 35 days
6 to 10 hours after surgery once hemostasis has been established,
with or without food, in patients with CrCl ≥15 mL/min
Avoid use in patients with CrCl <15 mL/min
Tablet shown not actual size.
After knee replacement surgery*†

10 mg
10 mg once daily for 12 days
6 to 10 hours after surgery once hemostasis has been established,
with or without food, in patients with CrCl ≥15 mL/min
Avoid use in patients with CrCl <15 mL/min
Tablet shown not actual size.
*Calculate CrCl based on actual weight.
†Patients with CrCl <30 mL/min were not studied, but administration of XARELTO® is expected to result in serum concentrations of rivaroxaban similar to those in patients with moderate renal impairment (CrCl 30 mL/min to <50 mL/min).
†CrCl = creatinine clearance; DVT = deep vein thrombosis.
VTE prophylaxis
Acutely ill medical patients*†‡

10 mg
10 mg once daily
In hospital and after hospital discharge, for a total recommended duration of 31 to 39 days, with or without food, in patients with CrCl ≥15 mL/min
Avoid use in patients with CrCl <15 mL/min
Tablet shown not actual size.
*For VTE prophylaxis in acutely ill medical patients at risk for thromboembolic complications who are not at high risk of bleeding.
†Calculate CrCl based on actual weight.
‡Patients with CrCl <30 mL/min were not studied, but administration of XARELTO® is expected to result in serum concentrations of rivaroxaban similar to those in patients with moderate renal impairment (CrCl 30 mL/min to <50 mL/min).
CrCl = creatinine clearance; VTE = venous thromboembolism.
CAD
Reducing the risk of major CV events

2.5 mg
2.5 mg twice daily
With or without food, in combination with aspirin (75 mg-100 mg) once daily
No dose adjustment needed based on CrCl
Tablet shown not actual size.
CAD = coronary artery disease; CrCl = creatinine clearance; CV = cardiovascular.
PAD
Reducing the risk of major thrombotic vascular events in patients with PAD, including patients after LER due to symptomatic PAD

2.5 mg
2.5 mg twice daily
With or without food, in combination with aspirin (75 mg-100 mg) once daily
No dose adjustment needed based on CrCl
When starting therapy after a successful LER procedure, initiate once hemostasis
has been established
Tablet shown not actual size.
CrCl = creatine clearance; LER = lower extremity revascularization; PAD = peripheral artery disease.
Additional information
Dosing guide
Get the complete guide to dosing for XARELTO®