REFERENCES: 1. The EINSTEIN–PE Investigators, Büller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287-1297. 2. The EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499-2510. 3. Weitz JI, Lensing AWA, Prins MH, et al; for the EINSTEIN CHOICE Investigators. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211-1222. 4. Prins MH, Lensing AWA, Bauersachs R, et al; EINSTEIN Investigators. Oral rivaroxaban versus standard therapy treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;11(1):21. 5. Caroti KS, Becattini C, Carrier M, et al. Rivaroxaban versus apixaban for treatment of cancer-associated venous thromboembolism. TH Open. 2023:7(3):e206-e216. 6. Barco S, Schmidtmann I, Ageno W, et al; HoT-PE Investigators. Early discharge and home treatment of patients with low-risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban: an international multicentre single-arm clinical trial. Eur Heart J. 2020;41(4):509-518. 7. Peacock WF, Coleman CI, Diercks DB, et al. Emergency department discharge of pulmonary embolus patients. Acad Emerg Med. 2018;25(9):995-1003. 8. Supplement to: Weitz JI, Lensing AWA, Prins MH, et al; for the EINSTEIN CHOICE Investigators. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211-1222. 9. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J.
2020;41(4):543-603. 10. Khorana AA, Noble S, Lee AVY, et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost.
2018;16(9):1891-1894. 11. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496-520. 12. Farge D, Frere C, Connors JM, et al. 2022 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022;23(7):e334-e347. 13. Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer[published correction appears in Blood Adv. 2021 Apr 13;5(7):1953]. Blood Adv. 2021;5(4):927-974. 14. Streiff MB, Holmstrom B, Angelini D, et al. Cancer-associated venous thromboembolic disease, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Can Netw. 2021;19(10):1181-1201.
In the EINSTEIN DVT/PE trials
~98% of patients did not experience another DVT/PE4*
EINSTEIN DVT
2.1% (36/1731) with XARELTO®
vs3.0% (51/1718) enoxaparin and warfarin/VKA
HR=0.68 (95% CI: 0.44-1.04)1
EINSTEIN PE
2.1% (50/2419) with XARELTO®
vs1.8% (44/2413) with enoxaparin and warfarin/VKA
HR=1.12 (95% CI: 0.75-1.68)2
*Patients were followed for an average length of treatment of 208 days in clinical studies.
CI = confidence interval; DVT = deep vein thrombosis; HR = hazard ratio; PE = pulmonary embolism; VKA = vitamin K antagonist.

XARELTO® vs Eliquis®* in VTE patients with cancer5
Recurrent VTE or bleeding-related hospitalization with XARELTO® vs Eliquis®
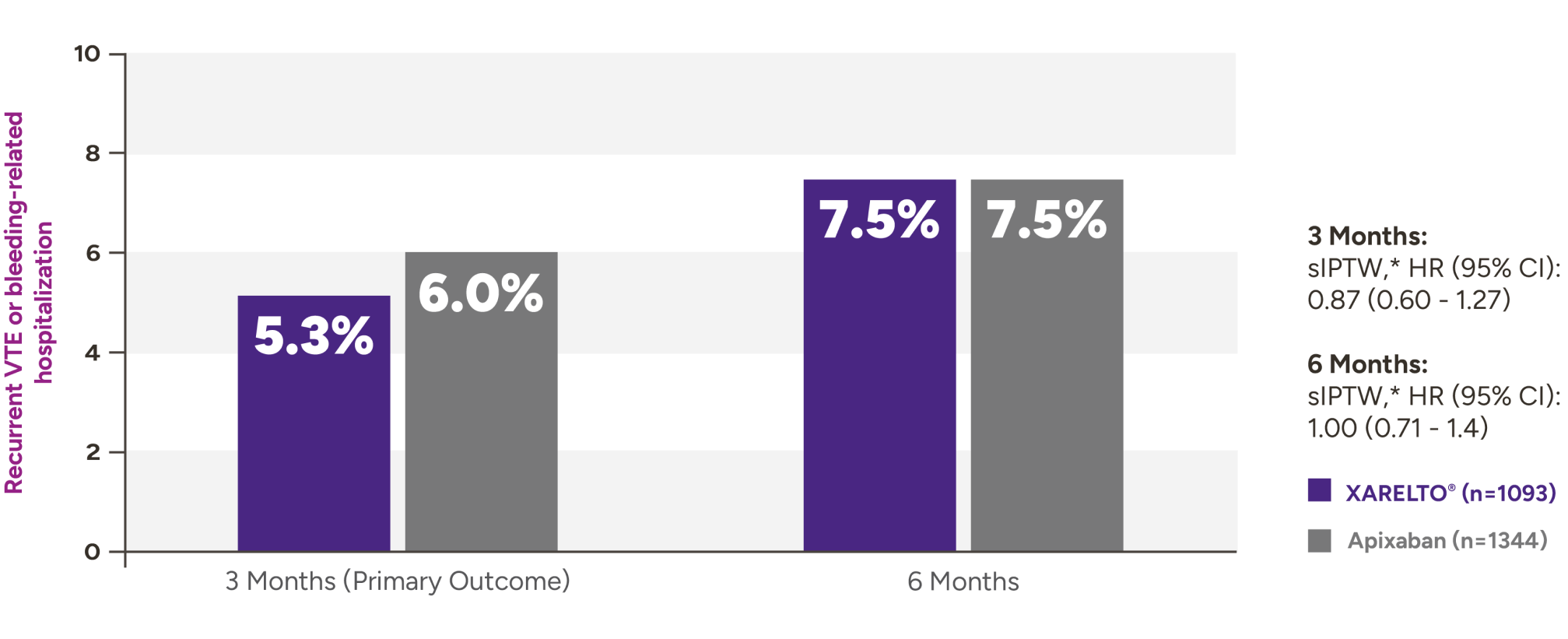
Secondary outcomes for VTE patients with cancer at 3 months and 6 months
| Outcomes at 3 months | XARELTO®n=1093,% | Eliquis®n=1344,% | sIPTW† | HR (95% CI) |
|---|---|---|---|---|
| Recurrent VTE | 3.8 | 4.2 | 0.92 (0.59-1.42) | |
| Bleeding-related hospitalization | 2.4 | 2.3 | 1.05 (0.59-1.88) | |
| Critical organ bleed | 0.2 | 0.4 | 0.49 (0.09-2.59) | |
| Recurrent VTE or critical organ bleed | 3.8 | 4.5 | 0.85 (0.56-1.31) | |
*Eliquis® (apixaban) is a registered trademark of Bristol-Myers Squibb Company.
†Propensity score model for sIPTW included demographics, laboratory values, clinical observations, comorbidities, cancer type, systemic cancer treatments, and concomitant noncancer medications.
CI = confidence interval; HR = hazard ratio; RWE = real-world evidence; sIPTW = stabilized inverse probability of treatment weight; VTE = venous thromboembolism.
In the HoT-PE trial
XARELTO® is the only DOAC to show safe and effective outpatient treatment in patients with low-risk PE6
>99% of patients with low-risk PE did not experience another event
0.6% (3/525) of patients with low-risk PE experienced another VTE or fatal PE when discharged early (P<0.0001)*
- Patients could have been treated with UFH, LMWH, fondaparinux, XARELTO®, or apixaban before study enrollment
- Protocol mandated that patients be discharged from the hospital within 48 hours after initial PE diagnosis
- Patients were followed for 3 months
*The majority of the exclusion criteria used to identify LRPE was adapted from the Hestia criteria.
DOAC = direct oral anticoagulant; LMWH = low-molecular-weight heparin; LRPE = low-risk pulmonary embolism; PE = pulmonary embolism; UFH = unfractionated heparin; VTE = venous thromboembolism.
In the MERCURY PE trial
XARELTO® significantly reduced hospitalization time with no observed differences in efficacy or safety in patients with low-risk PE7*†
Significant reduction in hospitalization for DVT/PE or bleeding events at 30 days in the MERCURY PE trial
33.6 hours
Standard of care
4.8 hours
Early ED discharge with
XARELTO®
Mean difference >1 day–28.8 hours
95% CI (-41.5 hours–16.2 hours) P<0.0001
*The MERCURY PE study was not statistically powered to evaluate efficacy and safety.
†LRPE was defined by the absence of any Hestia criteria and was adapted for emergency medicine by removing any 24-hour requirements.
CI = confidence interval; DVT = deep vein thrombosis; ED = emergency department; LRPE = low-risk pulmonary embolism; PE = pulmonary embolism.
In the EINSTEIN CHOICE trial
Superior reduction in the risk of recurrent DVT/PE3*
Recurrent DVT/PE with extended
treatment
74% RRR†
vs aspirin3.2% ARR‡HR=0.26 (95% CI: 0.14-0.47) P<0.001 (n=2258)
1.2% (13/1127) with XARELTO®
vs
4.4% (50/1131) with aspirin
Rates of recurrent DVT/PE in patients with a PE index event3
PE
0.4% (4/1127) with XARELTO®
vs
1.2% (14/1131) with aspirin
DVT
0.3% (3/1127) with XARELTO®
vs
0.6% (7/1131) with aspirin
DVT
and
PE
0.1% (1/1127) with XARELTO®
vs
0.0% (0/1131) with aspirin
Fatal VTE
0.0% (0/1127) with XARELTO®
vs
0.2% (2/1131) with aspirin
Nearly 50% of patients had a PE or DVT and PE as the index event in EINSTEIN CHOICE
Patients with BMI ≥30 kg/m2 experienced consistent rates of DVT/PE reduction8
Rates of DVT/PE in patients with a BMI ≥30 kg/m2
1.6% (6/376) with XARELTO®
vs
3.7% (14/375) with aspirin
NOTE:
Safety was not reported in BMI ≥30 kg/m2.
*After ≥6 months initial treatment.
†RRR calculated using 1 minus HR.
‡ARR = risk (placebo) - risk (experimental).
ARR = absolute risk reduction; BMI = body mass index; CI = confidence interval; DVT = deep vein thrombosis; HR = hazard ratio; PE = pulmonary embolism; RRR = relative risk reduction; VTE = venous thromboembolism.
In the EINSTEIN DVT/PE trials
Proven safety profile in initial DVT/PE treatment1,2,4
EINSTEIN PE1*
Rates of composite CRNMB and major bleeding
10.3% (249/2412) with XARELTO®
vs
11.4% (274/2405) with enoxaparin and warfarin/VKA
HR (95% CI): 0.90 (0.76-1.07)
Rates of major bleeding
51% RRR†
1.1% ARR‡
vs
enoxaparin and warfarin/VKA
HR=0.49 (95% CI: 0.31-0.79)§
1.1% (26/2412) with XARELTO®
vs
2.2% (52/2405) with enoxaparin and
warfarin/VKA
EINSTEIN-DVT2*
Rates of composite CRNMB and major bleeding
8.1% (139/1718) with XARELTO®
vs
8.1% (138/1711) with enoxaparin and warfarin/VKA
HR=0.97 (95% CI: 0.76-1.22)
Rates of major bleeding
0.8% (14/1718) with XARELTO®
vs
1.2% (20/1711) with enoxaparin and warfarin/VKA
HR=0.65 (95% CI: 0.33–1.30)§
EINSTEIN-DVT and PE4
Rates of composite CRNMB and major bleeding in pooled analysis
9.4% (388/4130) with XARELTO®
vs
10.0% (412/4116) with enoxaparin and warfarin/VKA
HR 0.93 (95% CI: 0.81-1.06)
Rates major bleeding in pooled analysis
46% RRR†
0.8% ARR‡
vs
enoxaparin and warfarin/VKA
HR=0.54 (95% CI: 0.37-0.79)§
1% (40/4130) with XARELTO®
vs
1.7% (72/4116) with enoxaparin and warfarin/VKA
*The principal safety outcome was the composite of major bleeding and clinically relevant nonmajor bleeding across treatment groups.
†RRR calculated using 1 minus HR.
‡ARR = risk (placebo) - risk (experimental).
§Not adjusted for multiplicity.
ARR = absolute risk reduction; CI = confidence interval; CRNMB = clinically relevant nonmajor bleeding; DVT = deep vein thrombosis; HR = hazard ratio; PE = pulmonary embolism; RRR = relative risk reduction; VKA = vitamin K antagonist.
Rates of bleeding in patients with low-risk PE in HoT-PE6*
1.2% (6/519) of patients experienced major bleeding
(95% CI: 0.4%-2.5%)
*The majority of the exclusion criteria used to identify LRPE was adapted from the Hestia criteria.
CI = confidence interval; LRPE = low-risk pulmonary embolism; PE = pulmonary embolism.
In the EINSTEIN CHOICE trial
The ONLY DOAC used for extended treatment to demonstrate a major bleeding rate as low as aspirin in DVT/PE3*
Rates of major bleeding†
0.4% (5/1127) with XARELTO®
vs
0.3% (3/1131) with aspirin
HR=1.64 (95% CI: 0.39-6.84)
Rates of major bleeding in patients with a PE index event
0.4% (2/560) with XARELTO®
vs
0.4% (2/564) with aspirin
*After ≥6 months initial treatment.
†Major bleeding for XARELTO® was 0.4% (5/1127) versus 0.3% (3/1131) for aspirin and was defined as overt bleeding that was associated with a decrease in the hemoglobin level of ≥2 g/dL, led to transfusion of ≥2 units of red cells, occurred in a critical site, or contributed to death.
CI = confidence interval; DOAC = direct oral anticoagulant; DVT = deep vein thrombosis; HR = hazard ratio; PE = pulmonary embolism.
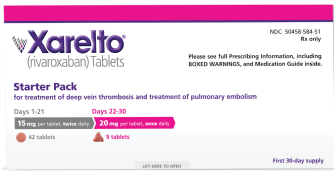
Dosing
For the treatment of
DVT and PE*

15 mg
For the first 21 days
15 mg twice daily with food, at the same time each day, in patients with
CrCl ≥15 mL/min
THEN

20 mg
Starting at day 22
Change to 20 mg once daily with food, at the same time each day, for remaining treatment in patients with CrCl ≥15 mL/min
Avoid use in patients with CrCl <15 mL/min
Tablets shown not actual size.
Consider the 30-day Starter Pack option for initial treatment of DVT/PE in adult patients


Once-daily* XARELTO®
(after BID initiation period of 21 days)
carries a lower pill burden than Eliquis®1,2
(after BID initiation period of 21 days)
carries a lower pill burden than Eliquis®1,2
*Taken with food.
Reduction in the risk
of DVT/PE recurrence*

10 mg
10 mg once daily
Taken with or without food after ≥6 months of standard
anticoagulant treatment in patients with CrCl ≥15 mL/min
Avoid use in patients with CrCl <15 mL/min
Tablet shown not actual size.
Tablet shown not actual size.
*Patients with CrCl <30 mL/min were not studied, but administration of XARELTO® is expected to result in serum concentrations of rivaroxaban similar to those in patients with moderate renal impairment (CrCl 30 mL/min to <50 mL/min).
BID = twice-daily; CrCl = creatinine clearance; DVT = deep vein thrombosis; PE = pulmonary embolism.

