REFERENCES: 1. XARELTO® [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals, Inc. 2. McCrindle BW, Michelson AD, Van Bergen AH, et al; UNIVERSE Study Investigators. Thromboprophylaxis for children post-Fontan procedure: insights from the UNIVERSE study. J Am Heart Assoc. 2021;e021765.
Oral-suspension delivery device
Can be used with all pediatric patients
Color-coded oral-suspension administration device designed to help with dosing and administration based on pediatric patient’s weight.
Side 1: dosing in milliliters (mL)

Side 2: dosing with color band (mL)*
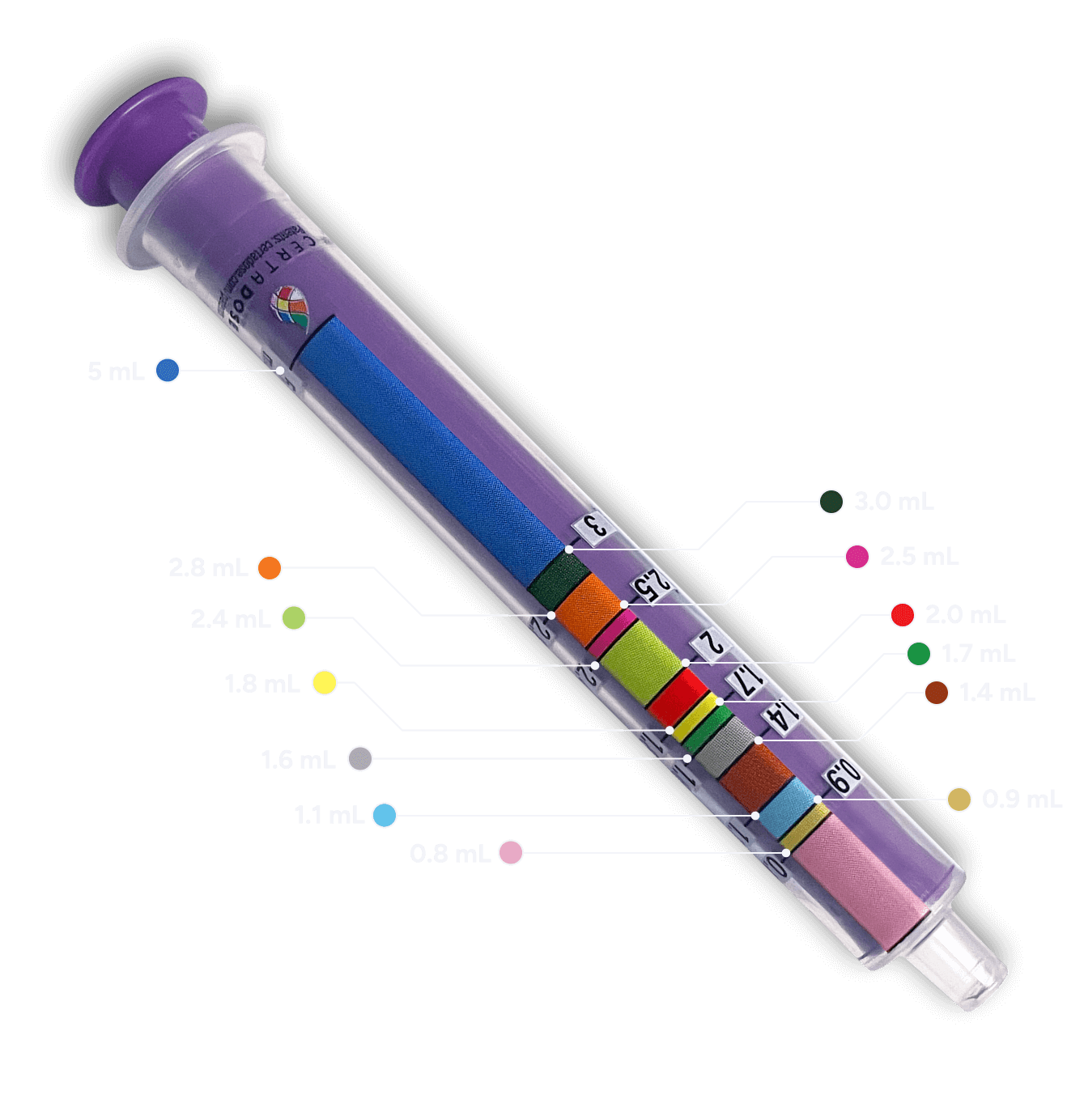
*The color band is an aid to withdraw the prescribed dose.
Pediatric weight-based dosing for VTE
Recommended dosing of XARELTO® for treatment of VTE and reduction in risk of recurrent VTE in patients up to <18 years of age1*†

Dosage:
1 mg XARELTO® = 1 mL SUSPENSION
Color-coding that corresponds to specific dosing markers on the oral syringe appears in the table.
| Body weight (kg) | Once a day‡ | 2 times a day‡ | 3 times a day‡ | Total daily dose§ |
|---|---|---|---|---|
| Oral suspension only | ||||
| 2.6 to 2.9 | 0.8 mg | 2.4 mg | ||
| 3 to 3.9 | 0.9 mg | 2.7 mg | ||
| 4 to 4.9 | 1.4 mg | 4.2 mg | ||
| 5 to 6.9 | 1.6 mg | 4.8 mg | ||
| 7 to 7.9 | 1.8 mg | 5.4 mg | ||
| 8 to 8.9 | 2.4 mg | 7.2 mg | ||
| 9 to 9.9 | 2.8 mg | 8.4 mg | ||
| 10 to 11.9 | 3.0 mg | 9 mg | ||
| 12 to 29.9 | 5.0 mg | 10 mg | ||
| Oral suspension or tablets | ||||
| 30 to 49.9 | 15 mg | 15 mg | ||
| ≥50 | 20 mg | 20 mg | ||
Administration in pediatric VTE patients1
Take with food
For the treatment of VTE in children, the dose should be taken with food to increase absorption.
Vomit or spit up?
- ≤30 minutes after receiving the dose: Give a new dose
- >30 minutes after the dose: Do not readminister the dose. The next dose should be taken as scheduled
- Vomits or spits up the dose repeatedly: Instruct caregiver to contact the child’s doctor right away
Administering tablets
- Do not split XARELTO® tablets in an attempt to provide a fraction of a dose
- If a child is unable to swallow a whole tablet, use XARELTO® oral suspension
- XARELTO® 2.5-mg tablets are not recommended for use in pediatric patients
Children <6 months of age
Dosing of XARELTO® was not studied in the following populations; therefore, dosing cannot be reliably determined and use is not recommended in children <6 months of age with any of the following:
- <37 weeks of gestation at birth
- <10 days of oral feeding
- Body weight <2.6 kg
Keep track of weight
To ensure a therapeutic dose is maintained, monitor the child’s weight and review the dose regularly, especially for children below 12 kg.

Please see the “Instructions for Use” in Prescribing Information for directions on using the oral-suspension formulation.
Download PDF*Initiate XARELTO® treatment following ≥5 days of initial parenteral anticoagulation therapy.
†Patients <6 months of age should meet the following criteria: at birth were ≥37 weeks of gestation, have had ≥10 days of oral feeding, and weigh ≥2.6 kg at the time of dosing.
‡Once a day: approximately 24 hours apart; 2 times a day: approximately 12 hours apart; 3 times a day: approximately 8 hours apart.
§All doses should be taken with feeding or with food since exposures match that of a 20-mg daily dose in adults.
VTE = venous thromboembolism.
Pediatric weight-based dosing post Fontan
Recommended dosage for thromboprophylaxis in pediatric patients with CHD after the Fontan procedure.

Dosage:
1 mg XARELTO® = 1 mL SUSPENSION
Color-coding that corresponds to specific dosing markers on the oral syringe appears in the table.
| Body weight (kg) | Once a day* | 2 times a day† | Total daily dose‡ | |
|---|---|---|---|---|
| Oral suspension only | ||||
| 7 to 7.9 | 1.1 mg | 2.2 mg | ||
| 8 to 9.9 | 1.6 mg | 3.2 mg | ||
| 10 to 11.9 | 1.7 mg | 3.4 mg | ||
| 12 to 19.9 | 2.0 mg | 4 mg | ||
| 20 to 29.9 | 2.5 mg | 5 mg | ||
| 30 to 49.9 | 7.5 mg | 7.5 mg | ||
| Oral suspension or tablets | ||||
| ≥50 | 10 mg | 10 mg | ||
Administration in pediatric post-Fontan patients1
Take with food
For thromboprophylaxis after Fontan procedure, the dose can be taken with or without food.
Administering tablets
- Do not split XARELTO® tablets in an attempt to provide a fraction of a dose
- If a child is unable to swallow a whole tablet, use XARELTO® oral suspension
- XARELTO® 2.5-mg tablets are not recommended for use in pediatric patients
Vomit or spit up?
- ≤30 minutes after receiving the dose: Give a new dose
- >30 minutes after the dose: Do not readminister the dose. The next dose should be taken as scheduled
- Vomits or spits up the dose repeatedly: Instruct caregiver to contact the child’s doctor right away
Keep track of weight
To ensure a therapeutic dose is maintained, monitor the child’s weight and review the dose regularly, especially for children below 12 kg.

Please see the “Instructions for Use” in Prescribing Information for directions on using the oral-suspension formulation.
Download PDF*Once a day: approximately 24 hours apart.
†2 times a day: approximately 12 hours apart.
‡All doses can be taken with or without food.
CHD = congenital heart disease.
Additional administration considerations
Dosing in pediatric patients with renal impairment
Patients ≥1 year of age
Degree of renal impairment
- Mild renal impairment (eGFR: 50 to ≤80 mL/ min/1.73 m2): no dose adjustment is required
- Moderate or severe renal impairment (eGFR: <50 mL/min/1.73 m2): avoid use, as limited clinical data are available
Calculating eGFR
- If SCr is measured by an enzymatic creatinine method that has been calibrated to be traceable to IDMS, eGFR can be calculated using the updated Schwartz formula: eGFR (Schwartz) = (0.413 x height in cm)/SCr in mg/dL
- If SCr is measured with routine methods that have not been recalibrated to be traceable to IDMS (eg, the traditional Jaffé reaction), the eGFR should be obtained from the original Schwartz formula: eGFR (mL/min/1.73 m2) = k x height (cm)/SCr (mg/dL), where k is the proportionality constant:
- k = 0.55 in children 1 year to 13 years
- k = 0.55 in girls >13 and <18 years
- k = 0.70 in boys >13 and <18 years
Patients <1 year of age:
Determine renal function using SCr. Avoid use of XARELTO® in pediatric patients <1 year with SCr results above 97.5th percentile, as no clinical data are available.
Reference values of serum creatinine in pediatric patients <1 year of age
| Age | 97.5th percentile of creatinine (mg/dL) | 97.5th percentile of creatinine (μmol/L) |
|---|---|---|
| Week 2 | 0.52 | 46 |
| Week 3 | 0.46 | 41 |
| Week 4 | 0.42 | 37 |
| Month 2 | 0.37 | 33 |
| Month 3 | 0.34 | 30 |
| Month 4-6 | 0.34 | 30 |
| Month 7-9 | 0.34 | 30 |
| Month 10-12 | 0.36 | 32 |
eGFR = estimated glomerular filtration rate; IDMS = isotope dilution mass spectrometry; SCr = serum creatinine.
Pharmacokinetic considerations:
- Half-life of rivaroxaban in plasma in pediatric patients treated for VTE decreased with decreasing age
- An exploratory analysis in pediatric patients treated for VTE did not reveal relevant differences in rivaroxaban exposure based on gender
| Mean half-life | Adolescents | 4.2 hours |
| 2 to 12 years | 3 hours | |
| 0.5 to <2 years | 1.9 hours | |
| <0.5 years | 1.6 hours |
VTE = venous thromboembolism.


XARELTO® management
| Switching pediatric patients to XARELTO® | From warfarin | From anticoagulants other than warfarin |
Discontinue warfarin and start as soon as the INR is <2.5 to avoid periods of inadequate anticoagulation. |
| |
| Switching pediatric patients from XARELTO® | To warfarin | To anticoagulants other than warfarin |
| When transitioning from XARELTO® to an anticoagulant with rapid onset, discontinue XARELTO® and give the first dose of the other anticoagulant (oral or parenteral) at the time that the next XARELTO® dose would have been taken. |
Switching pediatric patients to XARELTO®
From warfarin
Discontinue warfarin and start as soon as the INR is <2.5 to avoid periods of inadequate anticoagulation.
From anticoagulants other than warfarin
- Start XARELTO® 0 to 2 hours prior to the next scheduled administration of the drug (eg, LMWH or non-warfarin oral anticoagulant) and omit administration of the other anticoagulant
- For UFH being administered by continuous infusion, stop the infusion and start XARELTO® at the same time
Switching pediatric patients from XARELTO®
To warfarin
- To ensure adequate anticoagulation during the transition, continue XARELTO® for ≥2 days after the first dose of warfarin
- After 2 days of coadministration, obtain an INR prior to the next scheduled dose of XARELTO®
- Coadministration of XARELTO® and warfarin is advised until the INR is ≥2.0
- Once XARELTO® is discontinued, INR testing may be done reliably 24 hours after the last dose
To anticoagulants other than warfarin
When transitioning from XARELTO® to an anticoagulant with rapid onset, discontinue XARELTO® and give the first dose of the other anticoagulant (oral or parenteral) at the time that the next XARELTO® dose would have been taken.
INR = international normalized ratio; LMWH = low-molecular-weight heparin; UFH = unfractionated heparin.
Missed dose
If taking XARELTO® QD
- The patient should take the missed dose as soon as possible once it is noticed, but only on the same day
- If this is not possible, the patient should skip the dose and continue with the next dose as prescribed
- The patient should not take two doses to make up for a missed dose
If taking XARELTO® BID
- The patient should take the missed morning dose as soon as possible once it is noticed
- A missed morning dose may be taken together with the evening dose
- A missed evening dose can only be taken in the same evening
- The next day, the patient should continue with their regular regimen
If taking XARELTO® TID
- The patient should skip the missed dose and go back to the regular dosing schedule at the usual time without compensating for the missed dose
- The next day, the patient should continue with their regular regimen
Administration in pediatric patients through an NG or gastric feeding tube
XARELTO® oral suspension
- The oral suspension may be given through NG or gastric feeding tube. After the administration, flush the feeding tube with water
- An in vitro compatibility study indicated that XARELTO® oral suspension can be used with PVC, polyurethane, or silicone NG tubing
Feeding after administration
For the treatment or reduction in risk of recurrent VTE in pediatric patients, the dose should then be immediately followed by enteral feeding to increase absorption.
BID = twice daily; NG = nasogastric; PVC = polyvinyl chloride; QD = once daily; TID = three times daily; VTE = venous thromboembolism.