XARELTO® 2.5 mg with aspirin* is expected to help prevent
ischemic events relative to bleeds caused in patients with PAD1,2†
*XARELTO 2.5 mg twice daily plus aspirin (100 mg in clinical trial; 75 to 100 mg in practice) once daily.
In the VOYAGER trial:
†A favorable balance of benefits and risk was indicated: Compared to placebo (patients on placebo received aspirin 100 mg daily), during 10,000 patient-years of treatment, XARELTO® 2.5 mg twice daily plus aspirin 100 mg once daily would be expected to result in 181 fewer primary outcome events and 29 more TIMI major bleeding events.1
CV = cardiovascular; MI = myocardial infarction; TIMI = thrombolysis in myocardial infarction.
In the VOYAGER trial:
Superior risk reduction in a composite of major CV and limb
events versus placebo plus aspirin 100 mg QD1,2*
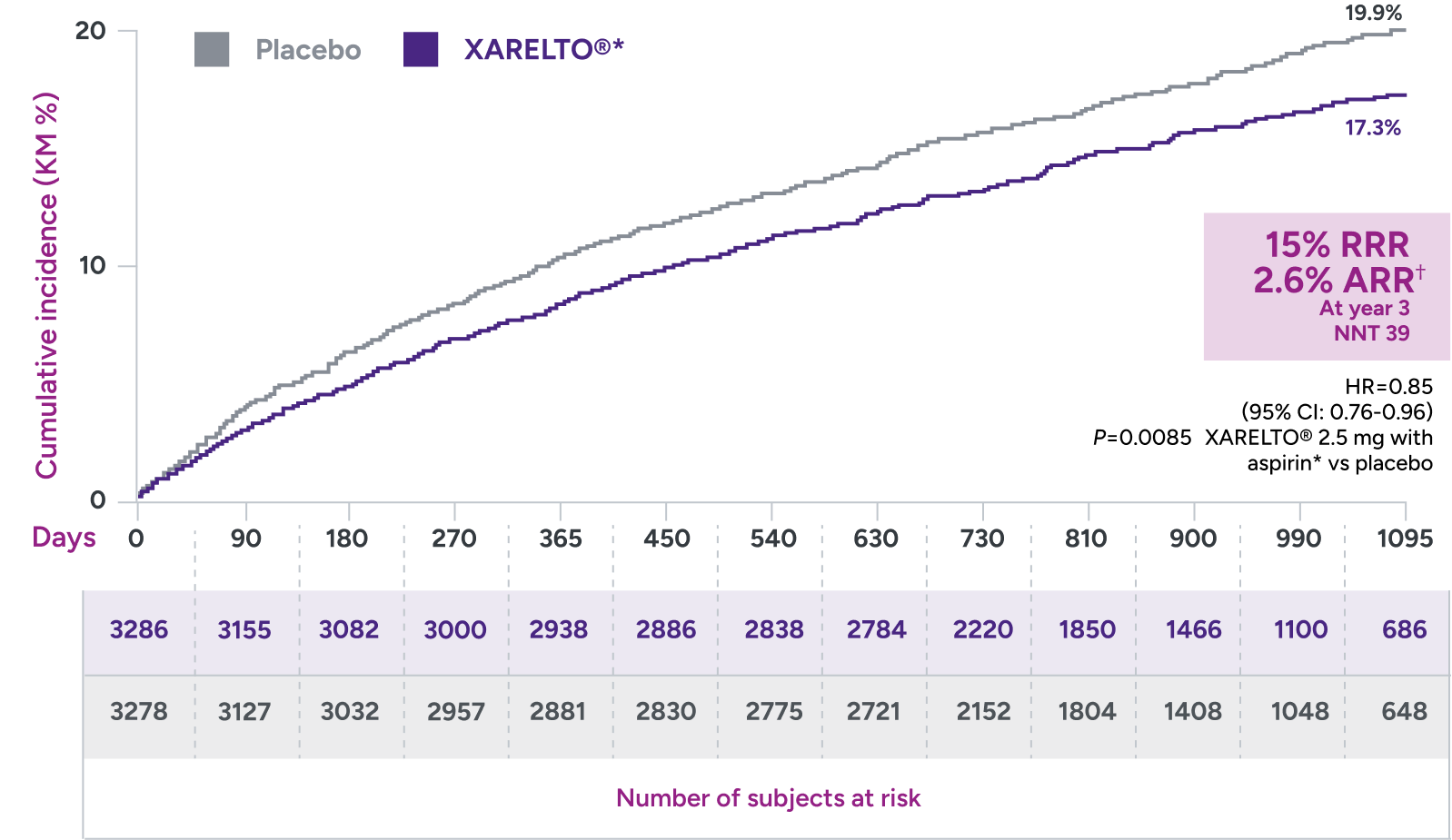
Primary endpoint:
A composite of ALI, major amputation for vascular cause, MI, ischemic stroke, and CV death
*Treatment schedule: XARELTO® 2.5 mg twice daily versus placebo; all patients received aspirin 100 mg once daily as background therapy.
†ARR = 19.9% - 17.3% = 2.6%.
ALI = acute limb ischemia; ARR = absolute risk reduction; CI = confidence interval; CV = cardiovascular; HR = hazard ratio; KM = Kaplan-Meier; MI = myocardial infarction; NNT = number needed to treat; QD = once daily; RRR = relative risk reduction.
Superior risk reduction in repeat revascularizations and rehospitalizations due to limb events versus placebo1,2*
Secondary efficacy outcomes in patients with PAD post LER
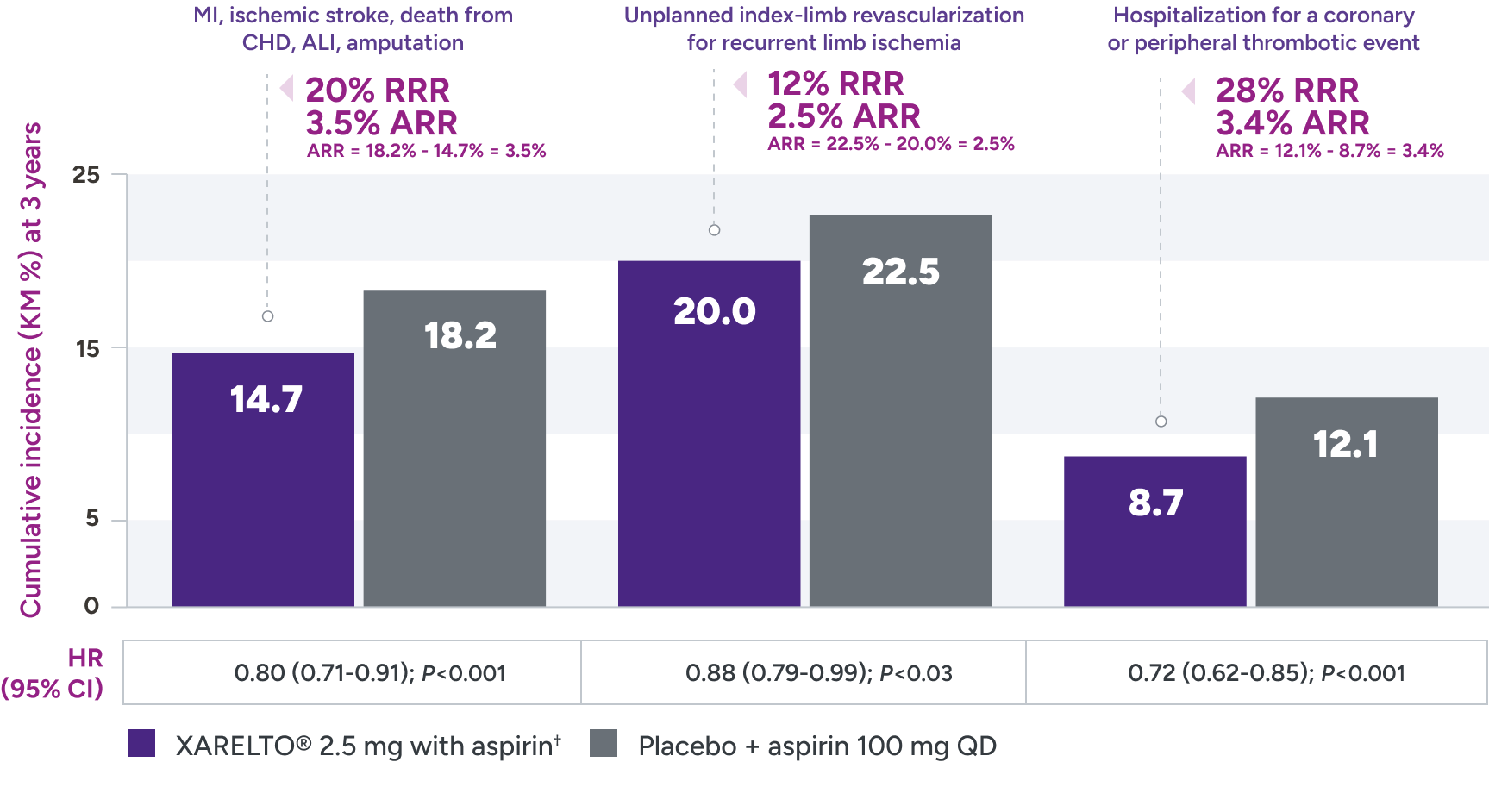
*Treatment schedule: XARELTO® 2.5 mg twice daily versus placebo; all patients received aspirin 100 mg once daily as background therapy.
ALI = acute limb ischemia; ARR = absolute risk reduction; CHD = coronary heart disease; KM = Kaplan-Meier; LER = lower extremity revascularization; MI = myocardial infarction; QD = once daily; RRR = relative risk reduction.
In the COMPASS trial:
Consistent results were demonstrated across major
thrombotic vascular events* in subgroup of patients with PAD2,3
Primary 5-component outcome:
Component outcomes for major thrombotic vascular events
QD
QD
QD: 2.0% (51/2492)
QD: 3.0% (67/2504)
QD: 1.0% (25/2492)
QD: 2.0% (47/2504)
QD: 3.0% (64/2492)
QD: 3.0% (78/2504)
QD: 1.0% (19/2492)
QD: 1.0% (34/2504)
QD: <1.0% (11/2492)
QD: 1.0% (28/2504)
*Reduction of a composite of myocardial infarction, ischemic stroke, cardiovascular death, acute limb ischemia and major vascular amputations.
†Not adjusted for multiplicity.
‡CV death includes coronary heart disease death, or death due to other CV causes or sudden cardiac arrest and unknown death.
§Acute limb ischemia was defined as limb-threatening ischemia with evidence of acute arterial obstruction by radiological criteria or a new pulse deficit leading to an intervention, such as surgery, thrombolysis, peripheral angioplasty, or amputation, within 30 days of symptoms onset.
||Investigator-reported events.
ARR = absolute risk reduction; BID = twice daily; CI = confidence interval; DOAC = direct oral anticoagulant; HR = hazard ratio; MACE = major adverse cardiovascular events; QD = once daily; RRR = relative risk reduction; TIMI = thrombolysis in myocardial infarction.
In the VOYAGER trial:
| XARELTO® 2.5 mg with aspirin† (n=3256) | Placebo‡ (n=3248) | |||||
|---|---|---|---|---|---|---|
| Outcome | PTs with event No. (%) | KM estimate at 3 yr (%) | PTs with event No. (%) | KM estimate at 3 yr (%) | HR (95% Cl) | |
| Principal safety outcome: TIMI major bleeding§ | 62 (1.90) | 2.65 | 44 (1.35) | 1.87 | 1.43 (0.97-2.10) | |
| Intracranial hemorrhage | 13 (0.40) | 0.60 | 17 (0.52) | 0.90 | 0.78 (0.38-1.61) | |
| Fatal bleeding | 6 (0.18) | 0.21 | 6 (0.18) | 0.21 | 1.02 (0.33-3.15) | |
| Intracranial or fatal bleeding | 17 (0.52) | 0.74 | 19 (0.58) | 0.97 | 0.91 (0.47-1.76) | |
| Secondary safety outcome | ||||||
| ISTH major bleeding‖ | 140 (4.30) | 5.94 | 100 (3.08) | 4.06 | 1.42 (1.10-1.84) | |
| BARC major bleeding¶ | 93 (2.86) | 3.86 | 73 (2.25) | 2.92 | 1.29 (0.95-1.76) | |
Principal safety outcome
No significant difference in TIMI major bleeding between XARELTO® 2.5 mg with aspirin† and placebo‡ was observed.
*
Treatment schedule: XARELTO® 2.5 mg twice daily versus placebo; all patients received aspirin 100 mg once daily as background therapy.
†
XARELTO® 2.5 mg twice daily plus aspirin 100 mg once daily.
‡
Patients on placebo received aspirin 100 mg once daily.
§
TIMI major bleeding is defined as fatal bleeding, intracranial hemorrhage, a decrease in hemoglobin level of ≥5 g/dL, or a decrease in hematocrit of ≥15%.
||
ISTH major bleeding is defined as fatal bleeding, bleeding into a critical site, a decrease in hemoglobin level of ≥2 g/dL, or transfusion of at least 2 units of packed red blood cells or whole blood.
¶
Bleeding Academic Research Consortium (BARC) major bleeding is defined as grade 3b or higher.
BARC = Bleeding Academic Research Consortium; CI = confidence interval; HR = hazard ratio; ISTH = International Society on Thrombosis and Haemostasis; KM = Kaplan-Meier PT = patient; TIMI = thrombolysis in myocardial infarction.
In a prespecified subgroup analysis of the VOYAGER trial, patients were administered clopidogrel at the investigator’s discretion7*
Absolute risk reduction† consistent with addition of XARELTO® 2.5 mg with aspirin,‡ regardless of clopidogrel use7§||
‡
XARELTO® 2.5 mg twice daily plus aspirin 100 mg once daily.
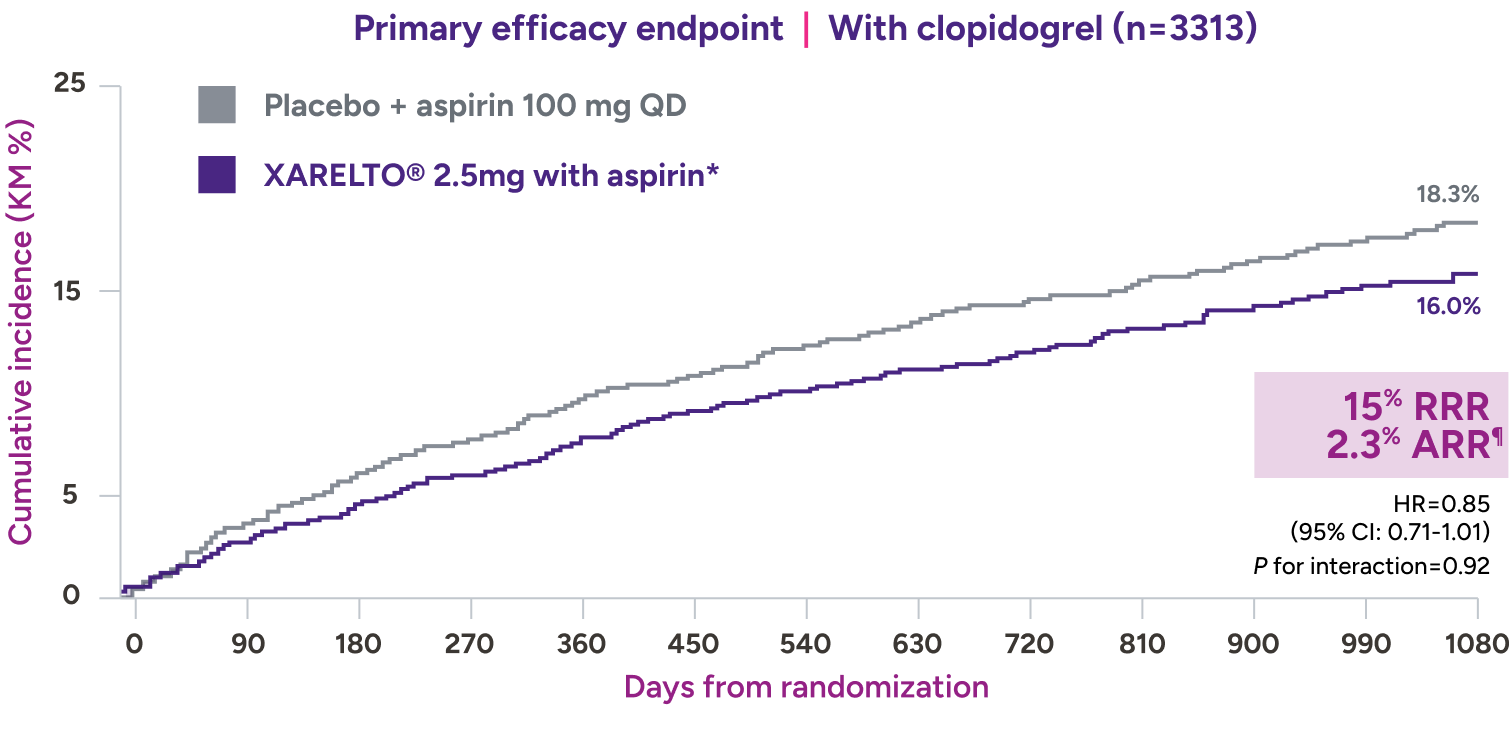
XARELTO® 2.5 mg with aspirin‡ consistently affected the risk of adverse CV and limb events,** with an early benefit for acute limb ischemia, regardless of clopidogrel use. Limb and CV risk reduction remained consistent over time.
With concomitant clopidogrel: 1.94% on XARELTO® and 1.47% on
placebo: HR=1.33 (95% CI: 0.78-2.26), ARI=0.5%‡‡
Without concomitant clopidogrel: 1.87% on XARELTO® and 1.25% on
placebo: HR=1.55 (95% CI: 0.88-2.72), ARI=0.6%§§
increase in ISTH major bleeding: HR=3.20 (95% CI: 1.44-7.13)
*
Clopidogrel use was not a randomized therapy and was associated with a different patient profile of CV risk, bleeding risk, and burden of concomitant CVD between the groups; direct comparisons of the efficacy and safety of clopidogrel in this setting are confounded, particularly without adjustment.
†
Reduction of a composite of myocardial infarction, ischemic stroke, acute limb ischemia, and major amputation of a vascular etiology in patients with PAD, including patients who have recently undergone an LER procedure due to symptomatic PAD.
‡
XARELTO® 2.5 mg twice daily plus aspirin 100 mg once daily.
§
Treatment schedule: XARELTO® 2.5 mg twice daily versus placebo; all patients received aspirin 100 mg once daily as background therapy.
||
Clopidogrel could be administered for up to 6 months.
¶
ARR = 18.3% - 16.0% = 2.3%.
#
ARR = 21.5% - 18.7% = 2.8%.
**
Composite endpoint: acute limb ischemia, major amputation for a vascular cause, myocardial infarction, ischemic stroke, or CV death.
††
TIMI major bleeding is defined as fatal bleeding, intracranial hemorrhage, a decrease in hemoglobin level of ≥5 g/dL, or a decrease in hematocrit of ≥15%.
‡‡
ARI = 1.94% - 1.47% = 0.5%.
§§
ARI = 1.87% - 1.25% = 0.6%.
ARI = absolute risk increase; ARR = absolute risk reduction; CI = confidence interval; CV = cardiovascular; CVD = cardiovascular disease; HR = hazard ratio; ISTH = International Society on Thrombosis and Haemostasis; QD = once daily; RRR = relative risk reduction; TIMI = thrombolysis in myocardial infarction.
COMPASS trial:
| Clinical endpoint | XARELTO® 2.5 mg BID + aspirin 100 mg QD %/year | (n/N) | Placebo (aspirin 100 mg QD) %/year | (n/N) | HR (95% CI) | Notes |
| Major bleeding* | 1.6 (263/9134) | 0.9 (144/9107) | 1.8 (1.5, 2.3) | |
| Fatal bleeding event | <0.1 (12/9134) | <0.1 (8/9107) | 1.5 (0.6, 3.7) | |
| Symptomatic bleeding in critical organ (nonfatal) | 0.3 (58/9134) | 0.3 (43/9107) | 1.4 (0.9, 2.0) | |
| Bleeding into surgical site requiring reoperation (nonfatal, not in critical organ) | <0.1 (7/9134) | <0.1 (6/9107) | 1.2 (0.4, 3.5) | |
| Bleeding leading to hospitalization (nonfatal, not in critical condition, not requiring reoperation) | 1.1 (188/9134) | 0.5 (91/9107) | 2.1 (1.6, 2.7) |
≤1%
of patients had fatal bleeding, nonfatal ICH, or critical organ bleeding5
*Major bleeding events within each subcategory were counted once per patient, but patients may have contributed events to multiple subcategories. These events occurred during treatment or within 2 days of stopping treatment in the safety analysis set.
BID = twice daily; CAD = coronary artery disease; CI = confidence interval; HR = hazard ratio; ICH = intracranial hemorrhage; PAD = peripheral artery disease; QD = once daily.
Dosing
Reducing the risk of major thrombotic vascular events in patients with PAD, including patients after LER due to symptomatic PAD

2.5 mg
2.5 mg twice daily
With or without food, in combination with aspirin (75 mg-100 mg) once daily
No dose adjustment needed based on CrCl
When starting therapy after a successful LER procedure, initiate once hemostasis has been established
Tablet shown not actual size.
CrCl = creatinine clearance; LER = lower extremity revascularization; PAD = peripheral artery disease.

